Pal International, a global leader in infection control products, and Mastermind Translations, a specialised language service provider for life science industries, have announced a strategic collaboration aimed at streamlining multilingual efforts for Pal International’s presence in over 50 overseas markets. This partnership was born out of a shared commitment to...
Airology theatre and treatment room for local hospital cuts patient wait times to four weeks
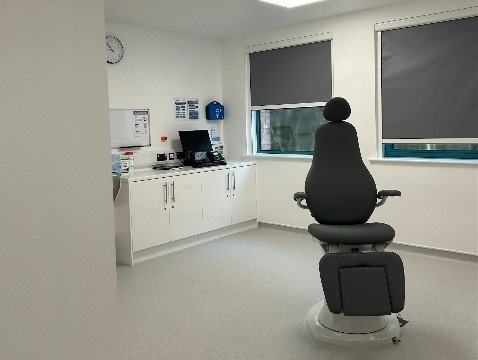
Expert cleanroom company Airology was commissioned to provide a new eye hospital for NHS patients who require cataract surgery and YAG laser capsulotomy in Worcestershire. The new hospital will help reduce waiting times for those requiring surgery to improve their vision, offering appointments to patients in just four to eight...
Important changes for Medilink Midlands
Life science industry organisation Medilink Midlands is making key changes to how it manages, supports and oversees its membership base. Changes in personnel will see Chris Dyke, Head of Connectivity and Innovation Adviser, Alison Morgan-Kimberley take a more proactive role in helping to support members and patrons. Chris has been...
IP Advance funding: What you need to know
A new, two-tiered funding programme launched by the UK’s Intellectual Property Office (IPO) promises to provide much needed support to SMEs looking to explore, or enhance, their intellectual property (IP) portfolios. Senior representatives from Gateley, including Medilink Midlands Patron Adamson Jones, provide a brief overview of the scheme. What is...
Pioneer Group’s Hexagon Tower spurs R&D surge in North Manchester
Three innovative R&D companies have expanded into the state-of-the-art Hexagon Tower in Blackley, signalling a growing interest in science infrastructure outside of Manchester city centre, according to site operator Pioneer Group. Hexagon Tower, part of Pioneer Group’s wider life sciences network that includes 645 R&D businesses across Europe, is a...
Airology supports Birmingham-based Xeal Pharma achieve ISO 7 multiroom cleanroom facility
Expert cleanroom company Airology was appointed to help design and build an ISO 7 cleanroom facility to enable Birmingham-based Xeal Pharma meet growing demands by creating a larger space for their production. The Process The client required an ISO 7 multiroom cleanroom facility with temperature control, specialist two room close...
Study reveals gene which slows growth and spread of pancreatic cancer is ‘switched off’ by disease
A new study has revealed that a gene which works to slow the growth and spread of pancreatic cancer is ‘switched off’ by the disease, allowing it to become more aggressive. Scientists at Nottingham Trent University, the University of Nottingham, Stanford University and the University of California and Cedars-Sinai Medical...
Growing the East of England Health and MedTech Cluster
Medilink Midlands is excited to announce that, we are expanding! With dedicated space at Arise Innovation Hubs Chelmsford, Essex, Medilink Midlands has joined the entrepreneurial ecosystem of innovators across the East of England. The Arise Innovation Hubs, part of Anglia Ruskin University (ARU) are designed to support collaboration with innovation pipelines,...
Smallfry joins Medilink Midlands as its latest Patron
Smallfry has further cemented its commitment to the life sciences sector by becoming a Patron of Medilink Midlands, a significant move underscoring their dedication to advancing medical technology and innovation. In addition to its proud membership with the West Midlands Health Tech Innovation Accelerator (WMHTIA), Smallfry has a proven history...
University of Nottingham spinout secures £500k for Tourette’s therapy wristband
Neupulse, a University of Nottingham spinout, has secured a 500,000 GBP equity investment through the Midlands Engine Investment Fund II, facilitated by appointed fund manager for the East and South East Midlands, Mercia Ventures. Founded in 2021 by husband and wife team, Professors Stephen and Georgina Jackson, with Dr Barbara Morera at the University...