Organisations from across the medtech and life sciences community have been recognised as finalists for the Medilink Midlands Business Awards 2025. The announcement was made at a Medilink Midlands networking event on 13 March, held in partnership with the West Midlands Health Tech Accelerator (WMHTIA). The event celebrated the achievements...
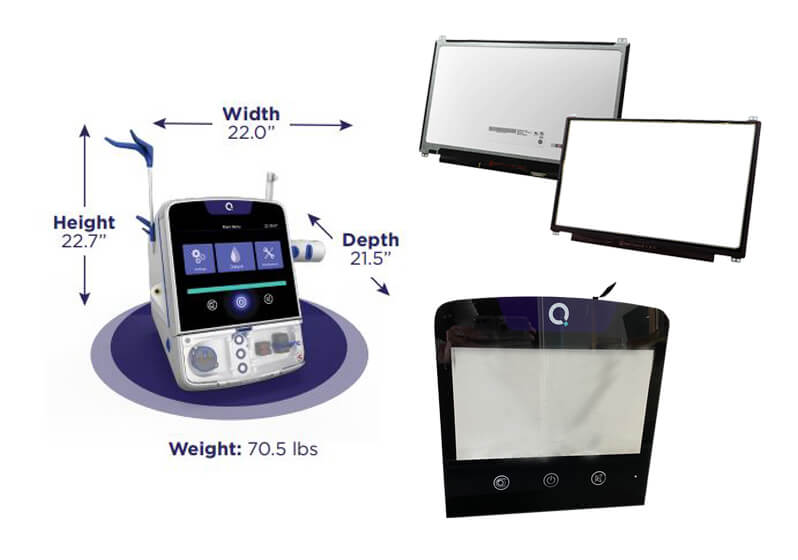
FORTEC UK provided TFT display for award winning portable Dialysis machine
NHS patients who suffer with kidney failure will now start to experience a more relaxed experience when connecting themselves to a dialysis machine. FORTEC UK was proud to support Quanta Dialysis Technologies, whose Quanta SC+ portable dialysis machine won the UK’s top engineering innovation award of 2022. This revolutionary device...
Blog: Why cleanrooms are essential for medical-grade cannabis companies
By Medilink Midlands Member Airology Systems The demand for medical-grade cannabis has surged in recent years, driven by its proven benefits in managing various health conditions. As this industry grows, maintaining the highest standards of quality, consistency, and safety becomes critical. For cannabis companies aiming to produce medical-grade products, implementing...
Australia’s Cannim partners with PHTA to advance medicinal cannabis research
Leading medicinal cannabis company Cannim has announced its UK operations will be based at Birmingham’s Precision Health Technologies Accelerator (PHTA), as part of a partnership aimed at furthering its research and improving patient outcomes. Cannim was established in Australia in 2017, curating products from across a network of EU GMP...
New sleep trial shows dramatic benefits for participants
Cambridge Sleep Sciences (CSS), a leading UK innovator in sleep technology, has announced groundbreaking results in a second trial of its pioneering SleepEngineTM technology, demonstrating the effectiveness of the technology in helping to improve the quality of adult sleep. The unique SleepEngineTM technology works by delivering a scientifically developed sequence...
Digital mental health technologies guidance launched to help manufacturers and safeguard users
The Medicines and Healthcare products Regulatory Agency (MHRA) has issued new guidance to help manufacturers meet UK medical devices regulations and ensure digital mental health technologies are effective, reliable and acceptably safe. From mental health apps, AI-powered assessments, and virtual reality therapy, digital mental health technologies are increasingly used by...
Scientific Laboratory Supplies’ service arm acquires new company
The dedicated service arm of Scientific Laboratory Supplies Group (SLS), C&M Scientific, has announced the acquisition of Radison Services Limited. This acquisition represents a significant step forward in C&M Scientific’s strategy to develop an industry-leading service brand, extending its geographical footprint across the UK and Ireland while reinforcing its commitment...
Golden Ticket Programme 2025 crowns winner
Melio Bio, a biotech dedicated to the discovery and development of novel inhibitors targeting a receptor implicated in obesity and associated co-morbidities, has been revealed as the winner of Pioneer Group and Novo Nordisk’s 2025 Golden Ticket Programme. The 2025 Golden Ticket Programme was open to early-stage biotech companies focused...
Illuminating biology: Advanced imaging and computational techniques transforming medical research at the University of Warwick
Advances in imaging and computational techniques are enabling researchers to discover more than ever before about health and disease at cellular and molecular levels, whether observing cell structures with ultra precision, imaging biological processes in real-time or studying disease mechanisms. Advanced imaging is crucial in applications ranging from diagnostics to...
From firefighting to future-proofing: Why predictive compliance is key in medical device software
By Medilink Midlands Member Coauthor by Hindsight In today’s fast-paced and highly regulated world of medical device development, the importance of staying ahead of compliance and security challenges cannot be overstated. Yet, many manufacturers still adopt a reactive, "firefighting" approach to these challenges. They wait until vulnerabilities or compliance gaps...